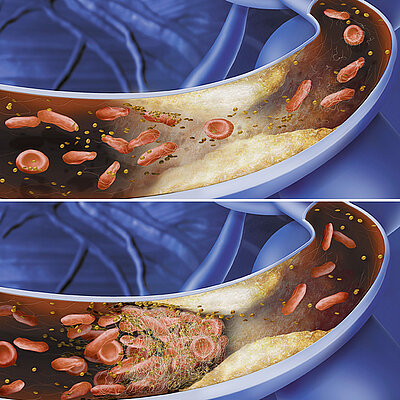
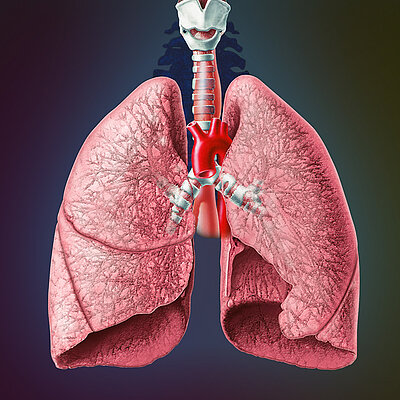
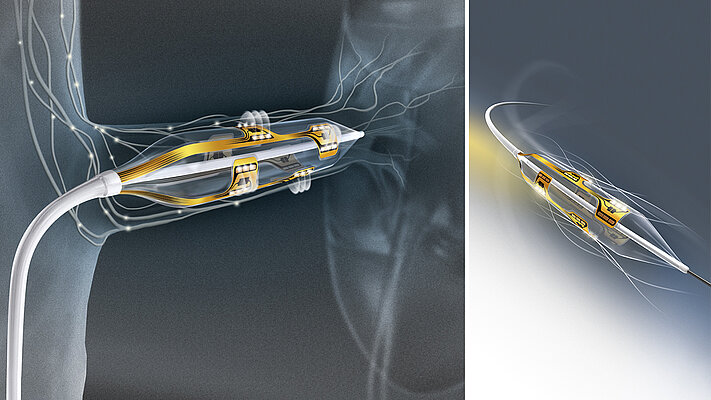
Boston Scientific invented a similar system, the Latitude-System, for patients with a cardiac pacemaker. Important data is sent by pressing a button from the pacemaker to the with the telephone signal connected Latitude Sytem, which will show the doctor the newest data – a classical method of telemedicine and remote supervision.
Pharma Relations (a leading german pharma magazine) plans a “digital cross-linking“ in 2015 for pharmaceutical companies. But this is the situation:
Data protection is important but it is also slowing down the development.
So basically, there are the digital-affine consumers/patients and the innovative mobile devices for supervising the health, the so called “Wearables“, for example Apple Watch, on the one hand. On the other hand, there are the new and strict measures of the FDA and a slow integration of digital media between the healthcare enterprises. A state, which could never be possible, in the FMCG-departement.
The technical possibilities could allow us a huge step forward, but the regulations put us two steps back. DeviceMed assumes that many providers will take their applications back because of the effort for the authorization.